Introduction
IEC 60601-1-2:2014 Edition 4 was published February 2014 and replaces IEC 60601-1-2 Edition 3 published on 2007. It pertains to EMC for medical electrical equipment and medical electrical systems. The European version (EN60601-1-2:2015) is identical to its IEC counterpart with exception of references to the EN versions of the 61000-4-x series and the addition of an Essential Requirements annex.
The motivation behind the 4th edition was to create a safety standard that pertains to electromagnetic disturbances in order to align with the general requirements of IEC 60601-1 Edition 3. The previous version of IEC 60601-1-2 did not adequately address the safety aspects as related to electromagnetic interference. In addition, the differences between edition 3 and 4 with respect to immunity are substantial.
Global Implementation
IEC 60601-1-2 Ed 4:2014 was published in February 2014. The FDA now recognizes the 4th edition and the mandatory compliance date for new submittals is December 31, 2018. That date was selected to harmonize with the requirements of the EU (EN 60601-1-2:2015). While not required until 2018, the FDA is currently accepting the 4th edition and prefers for products to be tested to that standard when submitting new applications, especially for devices to be used in the home healthcare environment.
The FDA does not require compliance to the 4th edition for legacy devices unless substantial changes are made to the product.
In the European Union, the Date of Withdrawal (DoW) of EN 60601-1-2:2007 is published as December 31, 2018. Therefore, all devices manufactured and imported into the EU after that date are required to comply with the 4th edition. There is no allowance for legacy devices as allowed by the FDA.
For other regions, the requirements vary significant by country. At least one major market does not currently accept the 4th edition. See Table 6 below for additional details.
Summary of Changes Between the 3rd and 4th Edition
The following significant changes were made for 4th edition.
- The classification of life support vs non-life support used in the third edition has been eliminated. It has been replaced with three intended use immunity categories. Professional Healthcare, Home Healthcare and Special Environment
- Modified emissions and immunity limits and levels as shown in Tables 1 through 4 below
- The immunity pass/fail criteria is limited to maintaining the Essential Performance and Basic Safety
- The Potential Equalization Conductor Terminal is required connected to the local ground during testing. The third edition make no mention of the Potential Equalization Conductor Terminal
- The use of the Artificial Hand is clarified
- The test methodology for performing ESD testing on connectors is modified
- The standby mode for both emissions and immunity testing should be considered
- A new procedure has been added to establish a procedure if a device is damaged during immunity testing
- Non-medical equipment used as part of the medical electrical system shall meet its relevant EMC requirements. The system shall maintain Essential Performance and Basic Safety
- The AC input voltage and frequency requirements are streamlined. With the exception of the Voltage Dips and Interruptions tests, testing with just one voltage & frequency within the device rated rage is acceptable
- Testing of SIP/SOPS (signal input/output ports) not used during patient use is clarified
- The allowance for radio susceptibly during radiated immunity testing has been eliminated.
- Labeling requirements modified & partially streamlined. The EMC Declaration of Conformity Tables listed in the third edition have been eliminated.
- An EMC test plan is required and includes recommended content
- The minimum dwell time used for immunity testing is reduced
- An EMC test report is required and minimum content is defined
- Risk Management – numerous EMC considerations are required
- All standards referenced are dated references (3rd edition uses undated references)
- The warning statement required in the instructions for use for Class A devices differs from that found in CISPR 11
- Devices used in aircraft should consider meeting the immunity requirements listed in RTCA DO-160
- The use of the ESD warning symbol near sensitive connectors has been eliminated.
Emissions Requirements
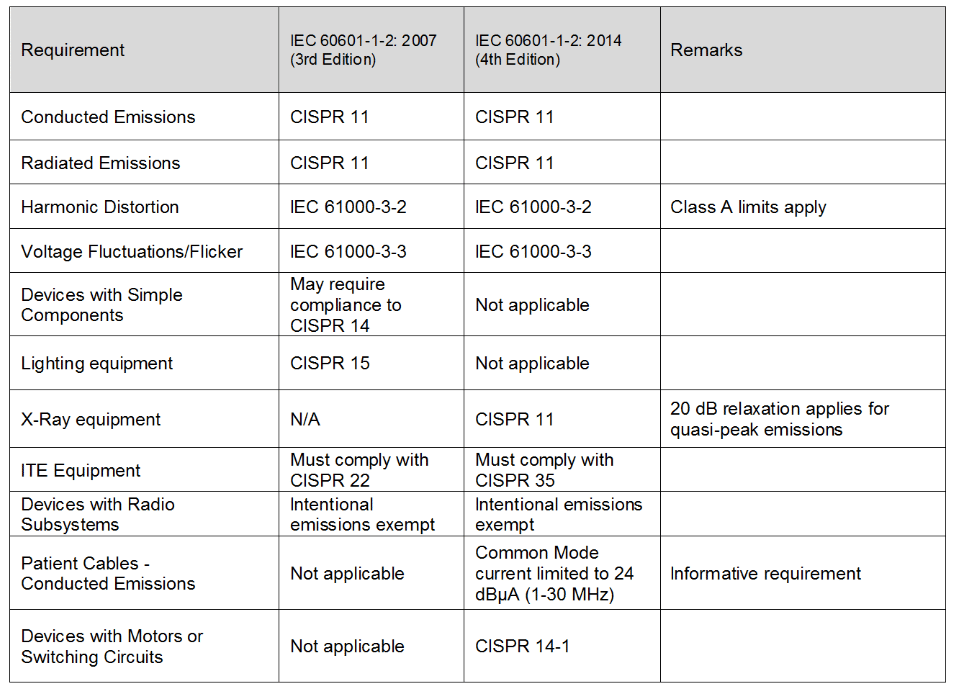
In most cases, the emission limits in the 4th edition are the same as those in the third edition. Various clarifications were made in the 4th edition as shown in Table 1 below.
Table 1- Emission Requirements Comparison
Immunity Requirements
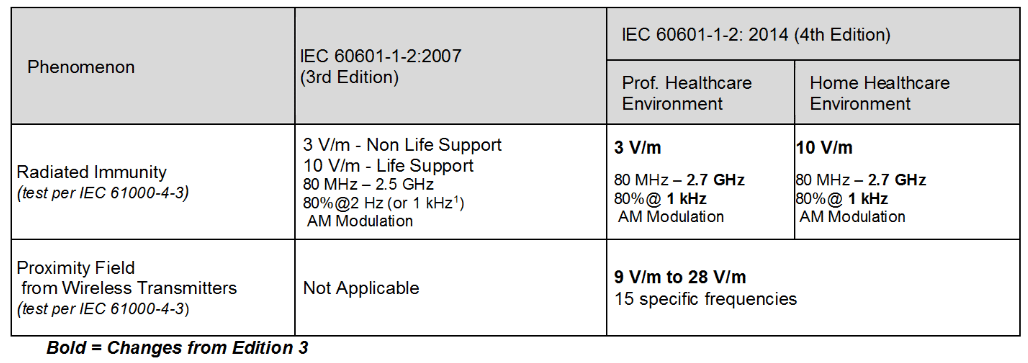
Significant changes for the immunity levels were made in the 4th edition as shown in Tables 2 through 4. The levels were based on “reasonably foreseeable maximum”.
Immunity Acceptance Criteria
The pass/fail immunity requirements detailed in the 4th edition is based on the medical device maintaining basic safety and Essential Performance. The definitions of those concepts are detailed below.
Basic Safety – As defined per IEC 60601-1:2012, Edition 3.1:
BASIC SAFETY
freedom from unacceptable risk directly caused by physical hazards when ME equipment is used under normal condition and single fault condition2
ESSENTIAL PERFORMANCE
performance necessary to achieve freedom from unacceptable risk performance of a clinical function, other than that related to basic safety, where loss or degradation beyond the limits specified by the manufacture results in an unacceptable risk.
NOTE – Essential Performance is most easily understood by considering whether its absence or degradation would result in an unacceptable risk.
Table 2- Immunity Level Comparison – Transient Phenomenon
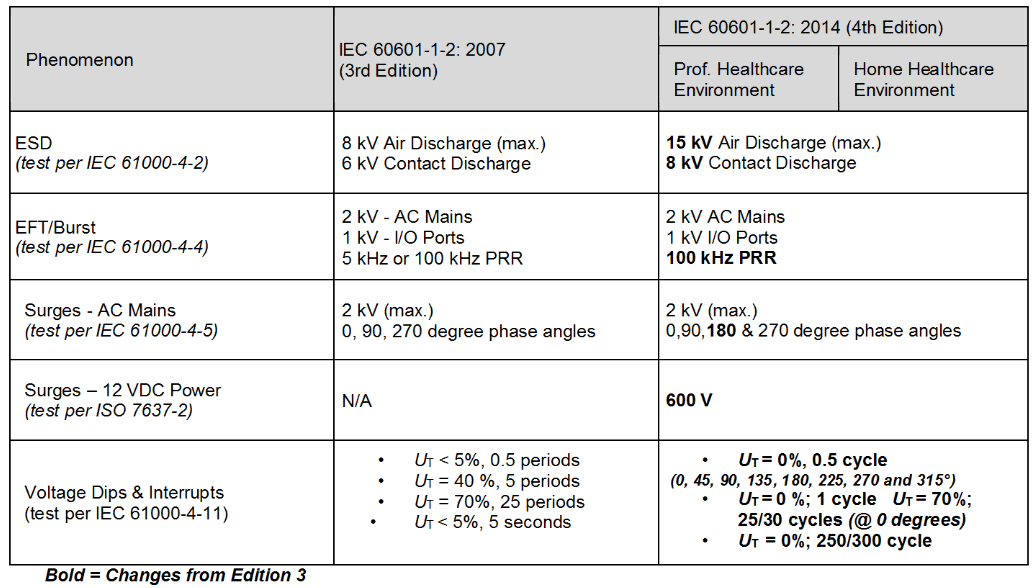 Table 3 – Immunity Level Comparison – Steady State Phenomenon
Table 3 – Immunity Level Comparison – Steady State Phenomenon
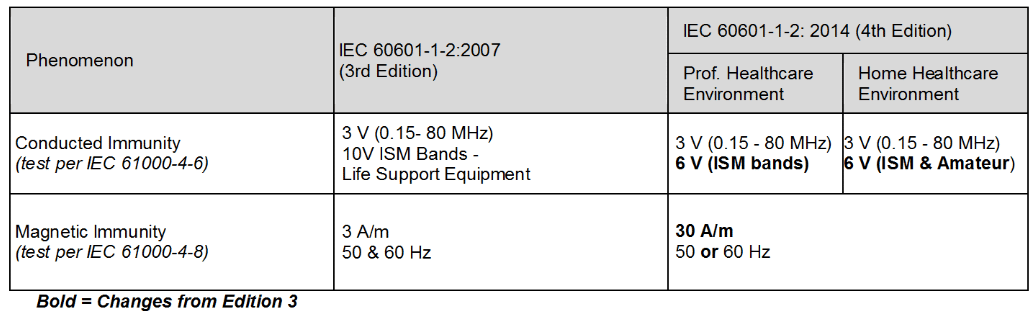
Table 4 – Immunity Level Comparison – Steady State Electric Field Phenomenon
 It is critically important for the manufacturer to understand and apply these above concepts for the medical device to be tested. In many cases, a device may be susceptible to electromagnetic disturbances, but if Basic Safety and Essential Performance are maintained, then the device may be judged as being compliant to the 4th edition.
It is critically important for the manufacturer to understand and apply these above concepts for the medical device to be tested. In many cases, a device may be susceptible to electromagnetic disturbances, but if Basic Safety and Essential Performance are maintained, then the device may be judged as being compliant to the 4th edition.
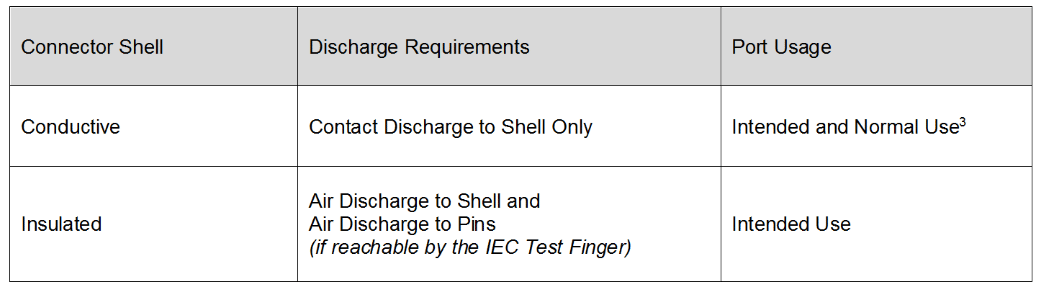
ESD Testing on Connectors
The third edition requires contact discharges be performed on individual connector pins that are accessible per the IEC guidelines of IEC 60601-1. There is no established test procedure to perform this test. The 4th edition refined this procedure per Table 5 below:
Table 5 – ESD Connector Testing Requirements
Risk Management
The concept of risk management for EMC is new. The 4th edition requires the following issues be addressed with respect to risk management. These items should be addressed and documented in the EMC test report and in the Risk Management file.
- The minimum separation distance with portable communication devices should be considered
- Annex F, Risk Management for Basic Safety and Essential Performance should be considered
- Operating modes used for testing should be based on Risk Analysis
- Testing of non-medical equipment (i.e. disturbances shall be taken into account in the risk management process)
- Effects observed of the EUT response during immunity testing should be addressed
- The Risk Management process shall be used to determine whether subsystem testing is allowed
- Reduced test levels, if used, must be justified
- Mitigations used to justify lower immunity test levels must be documented
- Alternative modulation frequencies can be used
- Current communications services shall be taken into account
- Must test to higher magnetic immunity levels if a device is closer than 15 cm to a power frequency magnetic source
Declaring compliance with the 4th edition requires five actions;
- A test plan be created prior to testing
- Compliance to relevant testing requirements
- A detailed test report with required minimum content specified
- The risk management requirements listed be addressed
- The device labeling be complaint per the requirements of clause 6.
Many test labs may be reluctant to assume the responsibility of reviewing the risk management and device labeling requirements. It is the responsibly of the manufacture to ensure all of the above items are adequately addressed.
Since publication, several errors and ambiguities have surfaced:
- There is discrepancy in the footnotes in Tables 1 and 5. Footnote c in Table 1 should be used.
- Table 9 is sometimes misread to require testing be performed at a 0.3 m test distance. The test distance as specified in IEC 61000-4-3 applies.
- The FDA does not accept the allowance listed in Table 8 Note b pertaining to cable length.
Dates for Compliance
The compliance dates to the 4th edition as well as many other standards can be a complex problem to solve. The dates are dependent of the type of device, the region it is sold into, and also may be driven by other higher level standards and regulations. Table 6 summarizes the mandatory compliance dates based on the region.
Table 6 – Compliance Dates for IEC 60601-1-2:2014 (4th edition) by Region

Summary
This article has provided a comparison of IEC 60601-1-2 3rd and 4th editions. As shown in the above tables, the test levels in many cases are different and are often (but not always) more stringent. The 4th edition requires the basic safety and essential performance of the medical device by maintained in the presence of the various electromagnetic environments listed in the standard. Susceptibilities not related to Basic Safety and Essential Performance would usually not be considered a test failure. In addition, the 4th edition requires of number of aspects be considered with respect to risk management.
The two standards are significantly different with respect to test levels and the immunity pass/fail acceptance criteria, the modes of operation, and more. Given this, it would be incorrect to state compliance with the 4th edition would equate to compliance with the third edition.
References
- 1 kHz modulation is used for medical devises that do not control, monitor or measure a physiological parameter.
- Although single fault condition is officially mentioned in this definition, many users of IEC 60601-1-2 would consider compliance during a single fault condition impractical to impossible to achieve and is therefore often ignored.
- Refer to IEC 60601-1 for the definition of intended and normal use.
- Per FDA substantial equivalence (predicate) scheme
- Some countries do not presently accept the 4th Edition
- The particular standards (IEC 60601/80601-2-X) are in process of converting to dated references to IEC 60601-1-2:2014. Until the conversion is completed, some of the part standards will reference IEC 60601-1-2:2014, some reference IEC 60601-1-2:2007 and others utilize an undated reference to IEC 60601-1-2.




