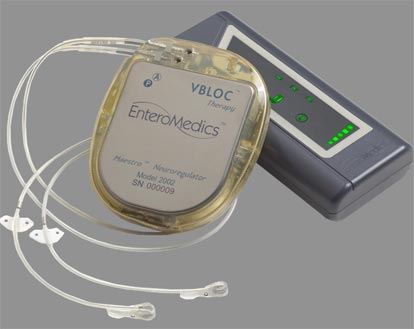
The Food and Drug Administration has approved a weight loss treatment device, the Maestro Rechargeable System, for obese adults. The device targets the nerve pathway between the brain and the stomach, which controls hunger and fullness feelings.
The Maestro Rechargeable System is the first FDA approved obesity device since 2007. The device is approved to treat patients 18 or older, with a body mass index (BMI) of 35 to 45, with one other obesity-related condition (such as type 2 diabetes), who have not been able to lose weight by any other means.
“The Maestro Rechargeable System consists of a rechargeable electrical pulse generator, wire leads and electrodes implanted surgically into the abdomen. It works by sending intermittent electrical pulses to the trunks in the abdominal vagus nerve, which is involved in regulating stomach emptying and signaling to the brain that the stomach feels empty or full,” according to the FDA.
The effectiveness and safety of this device were evaluated in a clinical trial including 233 patients with a BMI higher than 35. Seventy-six patients, who were in the control group, were compared to the other 157 patients, the experimental group. The experimental group received the active device and the control group received a device that was not active.
Results of the trial demonstrated that after 12 months, the experimental group lost 8.5 percent more excess weight than the control group. About half of the patients in the experimental group lost at least 20 percent of their excess weight and 38.3 percent of patients in the experimental group lost at least 25 percent of their excess weight.
Even though the experiment did not reach its goal, which was that the experimental group lose 10 percent more than the control group, the FDA Advisory Committee found the data is “supportive of sustained weight loss, and agreed that the benefits of the device outweighed the risks for use in patients who met the criteria in the device’s proposed indication.”
Benefits and risks of this device have both been reviewed by the FDA.
“As part of the approval, the manufacturer must conduct a five year post approval study that will follow at least 100 patients and collect additional safety and effectiveness data including weight loss, adverse events, surgical revisions and explants and changes in obesity-related conditions,” according to the FDA.