Co-Authors:
Leen-Pieter Deurloo | Martin Sas, Ph.D. | Marnik Vaes
Today’s electronics can generate strong electromagnetic interference (EMI) that may affect electromagnetic compatibility (EMC) and, as a result, impact device performance. Polymer compounds with integrated electrical conductivity offer a potential solution to resolve EMI and electrostatic discharge challenges. These thermoplastic materials deliver the optimum level of electromagnetic shielding for electronics, while providing exceptional freedom to design complex geometries and opportunities to streamline processing by avoiding secondary operations.
Conductive thermoplastic compounds can be used for electronics applications in automotive (e.g., advanced driver assistance systems, or ADAS); telecommunications, including 5G networks; consumer electronics; industrial solutions (e.g., robotics, power distribution); and healthcare (e.g., wireless diagnostic wearables).
Connected Medical Devices
Driven in part by the need to reduce costs, healthcare systems around the world are currently undergoing a paradigm shift in treating patients with acute and chronic conditions. They are increasingly moving care delivery from hospitals to outpatient and home settings. This transformation requires medical device manufacturers to integrate data acquisition and wireless connectivity functions into their products to enable remote patient monitoring and ultimately improve outcomes at lower treatment costs. This is the promise of connected devices.
Of course, connected medical devices must be demonstrably safe when used as designed, including in combination with other therapies, and in a range of different environments. Devices that require direct contact with patients—even simple skin contact—must also be biocompatible. They must function without interfering with, or impairing, basic immunological functions or causing allergic or toxic reactions.
The Challenge of EMI
Manufacturers of connected devices face an additional safety challenge—interference with the main content stream of the radio signals produced, which can potentially adversely affect device performance and accuracy. This situation is caused by the phenomenon known as electromagnetic interference (also called radio frequency interference, or RFI). It is the tendency of electronic devices in close proximity to affect each other’s operation in negative ways, such as creating signal noise. Electromagnetic interference has affected radio-based communications since the work of Guglielmo Marconi approximately 150 years ago. It remains a challenge for electronics, packaging and compliance engineers to this day.
There are three main mechanisms of EMI propagation: conduction, reactive coupling and radiation. Focus in this article is given to radiation in radio frequency (RF) spectra: a non-ionizing type of radiation.
Electromagnetic compatibility standards and testing aim to ensure that electrical devices can safely operate near each other with a minimum level of interference. Shielding, which isolates the device from its surroundings and from the signals of other devices, is often used to address the EMC requirements for managing EMI. In simple terms, shielding involves creating a type of Faraday cage around sensitive components within the device, which is usually made with a metal encasement or similar solution.
However, shielding can be a complex issue, as the majority of connected medical devices interact directly with wireless infrastructure or indirectly via a consumer device such as a smartphone or tablet. They rely on a range of RF bands with differing levels of signal power, and operate in a range of communication modes, primarily using short-range wireless technologies. Popular communication protocols used for and interacting with connected devices, including medical equipment, are near-field communications (NFC), Bluetooth (BT), WiFi (WLAN) and ZigBee, and low-power versions of these. They operate in license-free bands of the radio spectra such as industrial scientific and medical (ISM) and short-range devices (SRD). Each creates a need for shielding against EMI. Figure 1 shows the power performance for common wireless devices.
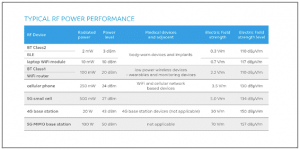
Figure 1: Typical RF power performance for common wireless devices expected to interfere with healthcare environments.
In general, EMI becomes significant at frequencies above 30 MHz, with typical levels of radiated emissions in units of electric field strength. Electromagnetic compatibility standards for healthcare-related and consumer electronics devices classify corresponding devices in a number of categories according to their intended use environment. They also define immunity levels of, and limits to, radiated EMI across a wide frequency range. Figure 2 summarizes some of these limits in comparison with the radiated power levels of certain wireless technologies.
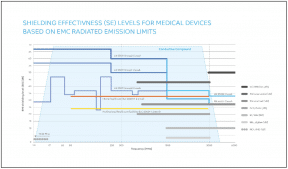
Figure 2: Electromagnetic compatibility-radiated emissions limits in terms of shielding effectiveness (SE) in dB based on EN 55011:2016, EN 55032:2015 and IEC EN 60601-1-2:2015 with examples of wireless technologies radiating RF power levels. The range of shielding effectiveness for a selected thermoplastic compound with inherent conductivity is highlighted.
Shielding effectiveness (SE) indicates the capacity of a material to act as a barrier against internal or external EMI and provide protection from the damaging signals of other electrical devices. It is determined by the material’s overall conductivity level, the wall thickness of the part and the target frequency range. Shielding effectiveness is typically measured with a dedicated setup of a vector network analyzer (VNA) for a specific frequency range and is determined by measuring scattering parameters.
Available thermoplastic grades for EMI shielding have an SE ranging from 33dB to 100dB. This SE range corresponds to shielding of more than 99.9 percent of signal power over a frequency range from 30MHz to 10GHz, at wall thicknesses from 1.2 mm and up. The EMI shielding capability of materials is typically measured according to the ASTM D 4935 standard.
New EMI Shielding Strategies
Conventional approaches to EMI shielding have relied on metal enclosures, usually made with aluminium alloys. This method currently accounts for more than half of the occurrences across relevant industries. However, the increasing miniaturization and engineering complexity of connected devices, along with demands to make them lighter and less intrusive, are posing challenges and highlighting design limitations. As weight becomes a greater consideration, even the lightest aluminium alloys are likely to be problematic, not to mention costly. One key factor is tooling cost: compared to an injection molding tool, a diecast aluminum tool has a significantly shorter lifespan (approximately 10x shorter). Further, the increasing sensitivity of these devices, combined with reductions in design space, could render them more susceptible to interference. Consequently, other solutions are needed to adapt to these evolving demands.
Some manufacturers have explored alternative approaches for providing shielding, such as applying metal coatings or conductive paints to the plastic or using vacuum deposition. While these methods can be acceptable, they can be less effective than metal enclosures and rely on secondary processes. Secondary steps add system costs and complexity and can increase the overall environmental footprint of the products through emission of volatiles. In addition, not all thermoplastics can withstand such treatments.
An alternative, innovative solution is using a thermoplastic compound that provides EMI shielding without secondary treatments like metal coatings. Such compounds deliver robust EMI shielding performance as an embedded, intrinsic property of the resin and the molded part. They incorporate conductive metal fibers that permeate the entire part and can prevent surface scratches or nicks from affecting shielding performance, as can occur with coatings.
Optimal dispersion of conductive fibers in the molded part is critical for maximum shielding performance. Figure 3a shows poor dispersal of the fibers; Figure 3b shows the optimized fiber dispersion vs. resin concentration.

Figure 3a: Scanning electron microscope (SEM) analysis illustrates inferior dispersion of conductive fibers in molded parts (PC resin).
Figure 3b: SEM analysis illustrates good dispersion of conductive fibers in molded parts (PC resin).
As well as simplifying delivery of shielding performance, conductive thermoplastic compounds provide additional benefits, as summarized in Figure 4. By avoiding the need for secondary processes, such as painting and metallization, these compounds offer manufacturers considerably wider design freedom. For instance, they can incorporate complex 3D shapes that would interfere with efficient application of paint.

Figure 4
The ability to use intricate geometries gives designers the freedom to further focus on patient care, rather than being limited by the requirements of metals and coatings. With the greater flexibility provided by thermoplastic compounds, designers can optimize ease of use, comfort and convenience. Further, these lightweight compounds can enhance device portability and ergonomics for patients and clinicians. Additionally, plastics allow manufacturers to use high-speed, high-volume molding processes that can reduce production costs.
Ensuring Patient Safety
Patient safety is a primary concern for medical device regulators. Optimizing safety through accurate and reliable device operation is one of the goals of EMI shielding. However, there is another safety aspect: biocompatibility. Device engineers and raw material specifiers may require the resin to undergo a range of tests to determine if its extractables could potentially cause harm to the human body. While biocompatibility is ultimately determined based on the final device, resin suppliers can help device manufacturers determine suitability of the resin in the early stages of development with an assessment of the most relevant sections of the full test battery, such as hemolysis, cytotoxicity, pyrogenicity or physico-chemical evaluation.
Expanding Industry Demand
The demand for connected medical devices will continue to grow rapidly for the foreseeable future. According to Mordor Intelligence, the connected medical devices market was valued at $23 billion USD in 2019 and is forecast to reach $77 billion by 2025. This growth is being propelled by the pressing need to constrain spiralling healthcare costs, as well as by advances in technology for measuring health parameters and performing monitoring functions. More connected devices, causing greater potential for interference, mean there will be even greater needs for effective shielding technologies and materials.
In such a dynamic environment, thermoplastic compounds with inherent EMI shielding properties can provide device manufacturers with cost-effective alternatives to traditional metal enclosures, coatings and paints. Specialty shielding compounds also deliver enhanced design freedom and enable weight reduction. The ability to create devices that are safe, biocompatible, more sustainable and lightweight, and feature more-innovative designs, can help increase their acceptance by patients and clinicians. In turn, patient adoption of such devices supports remote diagnosis, monitoring and treatment.
About the authors:
Leen-Pieter Deurloo is a business development manager for SABIC’s Specialties business in Europe. He is responsible for delivering high-performance compounds and copolymer solutions to customers in healthcare, telecommunications and mass transportation. He has more than 35 years of experience in technical and commercial roles in the plastics industry and is currently specifying electrical conductive materials for applications in the petrochemical industry. Leen holds a bachelor’s degree in chemistry from the HMLS Breda in the Netherlands.
Martin Sas, Ph.D., a lead scientist for SABIC’s Specialties business, manages projects and activities for electrical applications of high-performance polymers. His 15 years of experience include positions as R&D lead engineer for sensor development in the automotive industry, and system application engineer of embedded systems and MEMS sensors for automotive, healthcare and consumer electronics products. Martin holds a master’s degree in mechanical engineering and a doctorate in applied physics from Slovak Technical University in Slovakia.
Marnik Vaes is a business development manager for SABIC’s Specialties business in Europe. He helps to drive innovations in medical device platforms for multinational healthcare OEMs. He has more than 20 years of experience in application development and commercial roles focused on supporting customers in the healthcare industry. Marnik holds a master’s degree in industrial science from the University of Leuven, Belgium.