We are getting closer and closer: at the end of this year the new version of the standard concerning EMC tests and measurements on medical electrical equipment will become reality… yes, the old one will be superseded.
This change will influence (actually is already doing that) the whole approach to the EMC testing of these devices… on both sides! On one side, the EMC engineers. On the other, the manufacturers. In this article I will lay down all the differences between the previous and the last version of the standard, plus some electronic design hints for having a successful result on EMC testing against this new standard.
Manufacturers, please come forward!
If we carefully read the fourth edition of the standard, we notice that the manufacturer is deeply dragged into the measurement process, and not only that: his action begins even earlier, because he has to write the Test Plan for the entire measurement campaign, and he has to provide the plan to the Test Lab (standard reference, par. 6.2). But in order to do that, he has to understand deeply how measurements and tests are performed. If you keep on reading the standard, you will find that manufacturers will have to bear the heavy load, by issuing and writing a lot of documentation. The first document we are talking about is the Risk Management File, which is the final result of a process, denominated Risk Management Process.
Actually, there is not a specific guideline to help writing this document. Further in this article I will provide some hints about that. But why is the manufacturer more involved into the measurement process? Because the Risk Management File could state that, for instance, the conducted RF immunity should be using 45kHz of modulation frequency instead of the usual 1kHz. Or the same document could state that the minimum distance between the EUT and the RF wireless communication equipment is not 30cm but maybe only 10cm. Who will come in aid of the poor manufacturer?
Consultants, please come forward!
Believe me, the probability for you, the manufacturer, to have a hired skilled engineer able to draw a Test Plan for your medical device, is very low, at least in my country. So, this is really the fertile soil for consultants. In front of you, manufacturer, there are two ways, in order to tackle this new edition of the standard. First way: begin to read the standard, understand it, try to gain knowledge about EMC, and good design practices. I can suggest beginning with the main measurements and tests: from conducted RF emissions to Surges, the study of all of them will give a better knowledge to be aware of what your product will have to bear when it enters the doors of an EMC lab (“Abandon hope all ye who enter here” is not really reassuring…). Please use the internet: nowadays the Net is full of all sorts of hints and explanation about EMC tests, and often these kinds of resources are free of charge.
Second way: hire a consultant. I am sure that out there, there are lots of people, very skilled, and able to help you. Of course, they do not work for free, but the time to market will decrease compared to a self-made knowledge.
No pain, no gain: the main differences in terms of testing
Let’s get to the point. What are the differences between the third and the fourth edition of the standard? Do the immunity levels change? And what about the patient coupling ports? What if my device is to be installed on a vehicle? Patience, please: I will try to answer one question at a time.
Please have a look at the tables below: I have summarized the main differences.
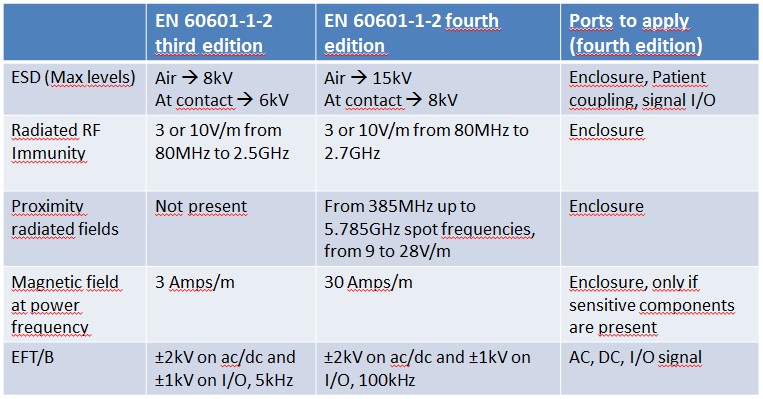
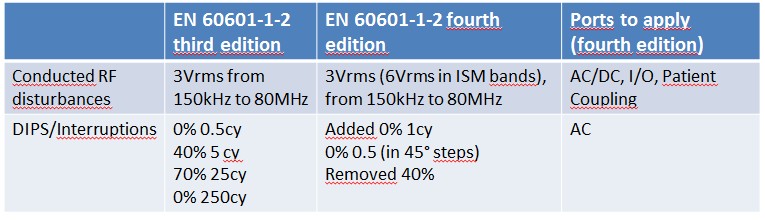

To cut short:
- New edition brings many changes: we are in front of almost an entire rewritten standard
- Some immunity levels have been increased (as an example, magnetic field from 3A/m to 30A/m), proximity wireless immunity was added
- Some immunity parameters and frequencies were changed (EFT/B frequency was changed from 5kHz to 100kHz)
- There are no more different types of patient coupling ports: the old definitions like F, BF, CF were removed
- For Large ME Equipment, a special chapter (8.6) gives some hints about “in situ” measurements
- For ME equipment intended to be installed on vehicles like ambulances, light commercial and passenger vehicles, tests are specified according to ISO 7637-2 (pulses on DC power supply, not required for EUTs inside the category Professional Healthcare Environment), and emissions according to CISPR 25
Tips and tricks
In this section I would like to give a few suggestions in order to minimize the “damage” eventually produced on your device by the new edition of this standard. I will focus mainly on ESD and the new Proximity Radiated fields. Let’s begin without wavering!
ESD: heavy task. For air discharges the level is almost doubled! Actually, there are two ways in order to pass ESD. The first is to avoid discharges: not very practical because at a level of humidity of 45%, the distance between the tip of the ESD gun and a metal plane is roughly 1cm for the spark to shoot (picture 1). If it is not possible to have such distance between, for instance a plastic case and a near metal part, please go for the second way: if you cannot avoid the discharge, just give it a safe low impedance path to follow, and a big, safe, metal surface to dissipate on.
Please be careful about displays: always connect to earth (or chassis) the metal frame around the display. And if the device does not have earth, provide big metal plane with conductive paint. If even this solution is not feasible, use capacitors (as explained in [2]): they are very useful especially on pins. And last but not least, do not let external exposed metal screws to penetrate inside your case… electrostatic discharge accumulates and suddenly discharges from the tip of the screw to the below nearer metal part.

Proximity Radiated fields: remember that the resonant frequency of a slot is given by the formula
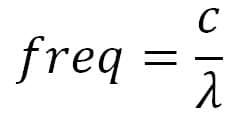
Where c is the speed of light in km/s and ? is the wave length expressed in m. EM fields as required by the standard, especially at higher frequencies (5 to 6GHz), can penetrate in slots with dimensions of some centimeters!
Annexes: please read them!
Maybe you wouldn’t expect to find important information (in some cases, even vital!) at the very, far end of a standard: actually, it is not the first example of this kind. EMC lab engineers work with standards every day: they really should know that technical committees are made of kind people, very generous in providing useful hints about immunity levels, general interpretation of the standard, and so on. Below I am providing a guide through the various annexes of this standard, so that with a quick glance you will be able to spot what you are looking for.
Annex B: about labelling and marking requirements
Annex C: how to classify your medical equipment (groups and classes according to CISPR 11)
Annex E: how to determine immunity levels for Special Environments
Annex F: about Risk Management (hints and tips). Please have a look at ISO 14971
Annex G: test plan. Please provide that to the Test Lab BEFORE the campaign!
The king of hints in EMC
After having written a lot of words about this big change in the EMC panorama of medical electrical devices, I think that only a few words can summarize the real kernel of all this. I will put them as a motto to be printed and shown in every R&D divisions. It goes like this: Knowing how a test or measurement is performed is the first step to avoid FAILS.
References:
[1] EN 60601-1-2:2014: https://webstore.iec.ch/publication/2590, fourth edition of the standard for EMC on medical electrical equipment
[2] http://electronicsbeliever.com/capacitor-as-esd-protection/, how to dimension a capacitor for ESD protection




