How does aging affect the shielding effectiveness of a gasket?
Brian W. Callen, Ph.D. and Claudia E. Johnston
Sulzer Metco (Canada) Inc., Fort Saskatchewan, Alberta, Canada
Nickel graphite conductive fillers have been replacing conventional silver-based fillers in EMI shielding gasket materials. Filled elastomer shielding gaskets include extrusions, molded shapes, die cut sheets, and form-in-place fabrication technologies. In addition to cost benefits, nickel graphite fillers have become preferred for applications where exposure to harsh industrial environments are known to be corrosive to silver-coated fillers. It is recognized that the primary advantage of silver or silver-coated fillers is the low interparticle contact resistance. Non-noble metals such as nickel invariably have thin non-conductive surface oxide layers that produce characteristically higher contact resistance.
Such differences in physicochemical properties of interparticle contact make non-noble metals more susceptible to natural degradation processes. The susceptibility of conductive fillers within elastomer binders to degradation processes of aging are typically evaluated in the laboratory through heat exposure testing. Common heat aging tests consist of exposing conductive elastomer sheets, molded parts or extrusions to elevated temperature for varying periods of times. Degradation caused by aging is usually evaluated by measuring the increase in volume resistivity of the heat aged material. Typical testing for commercial conductive elastomers is 150 °C for 48 hours.1 The increase in volume resistivity of a conductive elastomer from a heat-aging test is commonly used to qualify its stability for its particular application. Commercial standards commonly refer to a maximum allowable volume resistivity for conductive elastomers that have been subjected to a specific heat aging test.
Volume resistivity is a relatively simple and inexpensive measure of a conductive elastomer’s electrical properties, and a common tool for quality control. However, the measure of a conductive elastomer’s shielding eff ectiveness is recognized as the most relevant measure of its EMI shielding performance. Common methods of measuring shielding effectiveness, such as MIL-STD-285, are not routinely performed on shielding elastomers because of the expense, complexity, and inconvenience as compared to volume resistivity methods. Volume resistivity measurements do not account for the high frequency materials-dependant nature of EMI.2 Previous work on nickel graphite-filled elastomers has shown that changes in volume resistivity from conductive filler loading levels is not directly associated with changes in shielding effectiveness because of frequency dependence.3 A clear example of the material dependence of EMI shielding is illustrated by Kalinoski in which silvercoated glass and nickel-coated graphite are compared as conductive fillers for shielding elastomers.4 Kalinoski compared an elastomer with a silver-based filler to a nickel-graphite filled elastomer as both having a shielding effectiveness of at least 80 dB over a 10-MHz to 10-GHz range. However, those elastomers had volume resistivities of 1-50 mΩ·cm for the silver-based filler and 500–1000 mΩ·cm for the nickel-coated graphite filler. The example demonstrated that nickel graphite exhibited shielding effectiveness similar to that of silver although the volume resistivity of nickel graphite was 20–500 times higher. Kalinoski postulated that the “absorptive … properties of the graphite component of the filler synergistically interact with the electrical conductivity properties of the nickel component to attain an unexpectedly high level of EMI shielding effectiveness.”4 Similar examples of comparable shielding effectiveness between silver-based fillers and nickel graphite filler with contrasting volume resistivity can be found in conductive elastomer specification catalogs.1
Although the degradation processes of conductive elastomer aging have been documented in terms of volume resistivity, the effect on shielding effectiveness is difficult to find. It is known that volume resistivity in itself is an inadequate measure of the EMI shielding capabilities of conductive elastomers. The object of this paper is to present some data on the inter-relationships between aging, volume resistivity, and the shielding effectiveness of nickel-graphite filled conductive gaskets.
EXPERIMENT
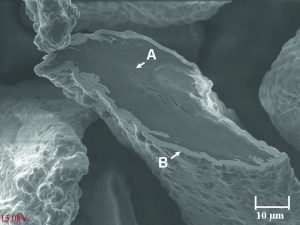
The conductive filler used in this work was nickel-coated graphite powder—weight composition 65% nickel and 35% graphite. The particle size range was 75 to 190 microns with an average particle size of 120 microns. The true particle density and apparent density of the nickel graphite powder was 4.3 g/cm3 and 1.39 g/cm3, respectively. Figure 1 shows a micrograph of the flake-shaped particles. The silicone elastomer used in this work was a commercially available heat-cured methylvinylpolysiloxane resin base that is a common type used in industry to produce EMI shielding gaskets. In the absence of conductive filler, the Durometer Shore A hardness of the elastomer was 30 as-cured and 46 as-postbaked and had a density of 1.1 g/cm3. The conductive filler was compounded with the silicone resin in a two-roll mill to 63.5% loading by weight prior to curing in a hydraulic hot press to form square 15-cm sheets, 1.7-mm thick. Following molding, each conductive rubber sheet was washed with isopropyl alcohol and then post-baked in an air-circulating oven. Two identical sheets were cut to produce 48 disks for volume resistivity measurements and six rings for coaxial shielding effectiveness measurements. The sheets were subsequently washed with isopropanol and allowed to dry prior to measurements. The cut disks (14-mm diameter, 1.7-mm thick) were measured for volume resistivity by the pressure probe method adapted from military specification MIL-G-83528B using a Keithley 580 four-point micro-ohmmeter. Coaxial shielding effectiveness measurements were conducted with a Spira™ ZT-1000 test fixture in conjunction with an Agilent™ 8560-E spectrum analyzer as described in previous work.3 The operational range of the test fixture was 20 MHz–1000 MHz. Sample rings were 6.35 cm in diameter and about 2.5 mm2 in cross-section. The rings were compressed by 20% in thickness by the brass contact flanges of the test fixture. Shielding is defined as the difference, in dB, between the signal voltage at the input of the test fixture with the sample in place and the signal voltage at the output with the sample in place in accordance with the SAE ARP 1705 procedure.5 Since the resulting measured shielding value depends on the circumference of the sample ring, the values were normalized to one meter. The normalized shielding effectiveness was thus calculated as:
Shielding effectiveness (dB) = Signal at input of test fixture with sample (dBm) – signal at output of test fixture with sample (dBm) + 20log(100/πd) (dB) (1)
where
d is the median diameter of the sample in centimeters.
Initial volume resistivity and shielding measurements were conducted (four disks and one ring accordingly) after post-baking and washing. The remaining disks and all rings were put into an air circulating oven set at 150 °C and ambient humidity. For each time point, four disks and one ring were removed from the oven and measured. Timepoint measurements were taken at 1, 2, 7, 14, 28, 41, 55, 71, and 114 days. Immediately following measurement, the ring would be returned to the oven for a subsequent time point measurement. Disks were used for one timepoint measurement only, and rings were used for two consecutive timepoint measurements before disposal.
Ring and disk samples for the day 114 measurement were re-measured following wiping the surfaces with a tissue soaked with isopropanol. The purpose of wiping with alcohol was to determine if the degradation caused by aging was associated with a removable surface layer or the bulk material.

RESULTS

Shielding effectiveness versus frequency is shown for 0 (initial) and 114 days (final) aging in Figure 2 between 20 MHz to 1000 MHz using the coaxial shielding test fixture. The noise limit for the instrument was 155 dB. The plots are smooth with frequency and show the decrease of shielding effectiveness with aging. The decrease in measured shielding effectiveness was 8 dB at 20 MHz, and approximately 15 dB at 100 MHz and higher. Shielding for the alcohol wiped 114 day sample decreased by a negligible amount of approximately dB (Figure 3b). This small difference (observed across most of the frequency range) indicates that the shielding was not significantly affected by wiping the surface with an alcohol-soaked tissue.
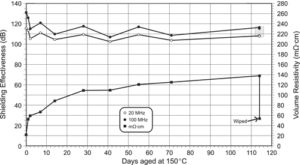
Volume resistivity and shielding effectiveness at 20 MHz and 100 MHz are plotted versus the number of days aged at 150 °C (Figure 3a). The decrease in shielding effectiveness sis most evident in the first two days of aging. At 100 MHz, the shielding effectiveness decreased from 131 to 116 dB over the 114-day test period. The shielding effectiveness data show an inherent time-dependant variability or noise level of approximately 10 dB. A similar trend is observed at 20 MHz and is off set from the 100-MHz plot by approximately 8 dB. The time-dependant variability has been attributed to slight differences in compression of the gaskets within the test fixture.
Volume resistivity increased most rapidly from day 0 (22 mΩ·cm) to day 1 (52 mΩ·cm), an increase of 30 mΩ·cm (Figure 3). By day 20 the increase in volume resistivity settled to a constant rate of approximately 0.33 mΩ·cm per day. By the end of the 114-day test period, the volume resistivity had risen to 138 mΩ·cm. The rapid initial increase correlates with the rapid initial decrease in shielding effectiveness over the same period. Te 114-day disk sample was subsequently wiped with an alcohol-soaked tissue and was remeasured for volume resistivity. As a result of wiping, the volume resistivity dropped from 138 mΩ·cm to 54 mΩ·cm, which is a value close to the day 1 measurement. Wiping the gasket surface with an alcohol-soaked tissue had a large effect in reversing the effect of aging in terms of volume resistivity. The volume resistivity data show that the aging process produces a non-conductive surface layer that can be removed by wiping with alcohol. Surface wiping the aged sample had the effect of recovering the volume resistivity to 72%. Although the effect was significant for volume resistivity, the presence of the non-conductive surface layer had a negligible effect on shielding effectiveness with a 1 dB decrease (Figure 3b). Previous unpublished work has shown that this surface layer does not form in an oxygen-free environment, so it cannot be associated with migration of silicone monomers or additives to the surface of the gasket. The surface layer involves an oxidation process and may be attributed to exposed nickel graphite having an aging-related oxide layer that is readily removed by wiping. This result is in contrast to the shielding effectiveness measurement, which did not detect a significant change from the same surface cleaning process.
Shielding eff ectiveness and volume resistivity were used concurrently as measures of gasket degradation caused by aging. It is evident that large changes in volume resistivity were not directly refl ected in shielding effectiveness. This is due, in part, to the logarithmic decibel scale of shielding effectiveness that is in contrast to the linear scale of volume resistivity. It is clear that surface eff ects have a larger role in the measurement of volume resistivity, as was revealed by the effect of cleaning the gasket with alcohol following aging. The sample that was cleaned with alcohol following aging recovered by 72%, whereas there was no detectable change in shielding eff ectiveness from the same cleaning process. It is evident that volume resistivity measurements in themselves can be misleading; in this case because of sensitivity to surface effects. Such surface eff ects are shown here to be less significant for characterizing the EMI shielding characteristics of the gasket.
CONCLUSION
Heat-aged nickel graphite filled elastomer gaskets were evaluated by volume resistivity and shielding effectiveness methods. The volume resistivity measurements were highly sensitive to changes in the surface condition of the gasket in contrast to the shielding effectiveness using a coaxial test fixture, which was more sensitive to bulk changes. After 114 days of aging at 150 °C, the nickel graphite filled elastomer provided greater than 100 dB of shielding effectiveness, which is more than sufficient for most applications.
REFERENCES
1. EMI Shielding for Commercial Electronics. Chomerics, Div. of Parker Hannifin Corp. Issued product catalog, 1997.
2. Thomas Clupper. “Correlating DC resistance to the shielding effectiveness of an EMI gasket.” ITEM 1999. Robar Industries, West Conshohocken, PA. 1999.
3. Brian Callen et al. “Influence of conductive filler loading on EMI shielding and DC volume resistivity.” Interference Technology Annual EMC Guide 2003. Robar Industries, West Conshohocken, PA. 2003. pp. 201 – 208.
4. Corrosion-Resistant, Form-in-Place EMI Shielding Gasket. John P. Kalinoski. US Patent 5910524, June 8, 1999.
5. “Coaxial Test Procedure to Measure the RF Shielding Characteristics of EMI Gasket Materials.” SAE ARP 1705, Issued 6-1-81. Reaffirmed 12-91. Society of Automotive Engineers, Inc.
ABOUT THE AUTHORS
Brian W. Callen, B. SC., PH.D. is a senior research and development professional for Sulzer Metco’s Electronic Materials Unit. In 1992 he received his doctoral degree in chemistry from the University of Western Ontario where he specialized in fundamental chemical processes of nickel surfaces. Prior to joining Sulzer Metco in 1999, he was employed as a scientist in product development at AMP Inc. where his activities focused on materials for electrical connection applications. He may be contacted at 780-992-5154 or Brian. Callen@sulzer.com.
Claudia E. Johnston is a research and development technologist for Sulzer Metco’s Electronic Materials Unit. Prior to joining Sulzer Metco in 2001, she had worked extensively for more than 10 years in the industrial sector and for the Department of National Defense providing real-time analytical process support and methodology development. She holds full member (MCIC) status in the Canadian Society for Chemical Technology.
