Tungsten carbide/aluminum composite particles inhibit galvanic corrosion of aluminum.
An on-going issue in the design of reliable EMC shielding is galvanic corrosion of aluminum. How can an aluminum frame be protected from galvanic corrosion if it contacts an EMI gasket containing non-aluminum particles? Chromate conversion coatings have long been used to reduce the corrosion rate of aluminum, but with RoHS restrictions on Cr (VI), the problem becomes more severe. A viable solution to this issue is an aluminum-based conductive filler for EMI gaskets that produces almost no aluminum corrosion. It contains no silver or nickel, yet it provides excellent shielding effectiveness with long-term stability. This article describes the mechanism of galvanic corrosion and how it affects gasket material selection.
GALVANIC CORROSION
Unlike oxidation, galvanic corrosion is a process that occurs by electron transfer between two dissimilar metals in a corrosive environment. In this situation, the natural oxidation characteristics of each metal are enhanced such that the metal that is more easily oxidized experiences a higher corrosion rate than it would were it not in contact with the other metal. The metal that is less prone to oxidation experiences a lower corrosion rate than normal.
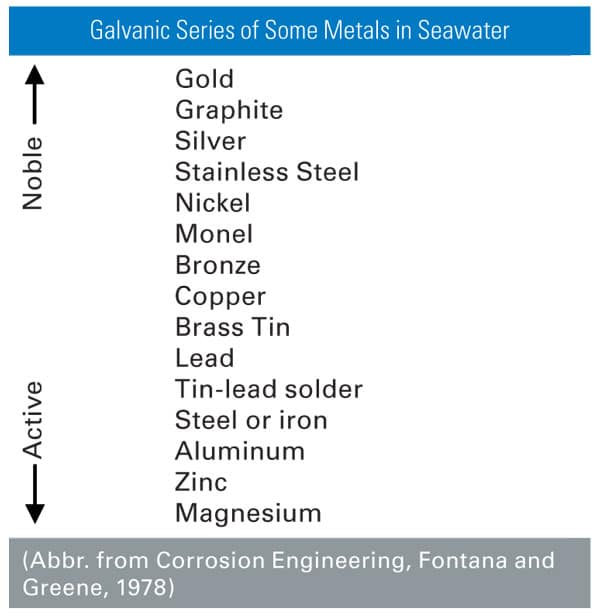
In general, a galvanic series chart of metals can be used to predict which metals will corrode faster or more slowly when placed into contact with each other (Figure 1). The positions of the metals on the chart indicate which are more noble (cathodic) and which are more active (anodic). The farther apart the metals are in the series, the greater the potential for galvanic corrosion.
Herein lies the problem. Aluminum is a very active metal—it readily forms a natural oxide layer. Metals often selected for EMI shielding are less prone to oxidation. Combine the two metals, and the potential for galvanic corrosion is created.
TYPICAL CONDUCTIVE FILLERS
Elastomer gaskets for EMI shielding are typically filled with metal or metal-coated particles that offer high conductivity. Silver-coated particles are often chosen for applications where high performance and long-term stability is required since silver is the most conductive metal and even its oxide is somewhat conductive. Based on the galvanic series chart, if silver is put into contact with aluminum in a corrosive environment, the aluminum corrosion rate will significantly increase because of galvanic coupling. For this reason, some manufacturers have chosen nickel or nickel-coated graphite as a non-silver alternative filler. Research shows, however, that this can cause more aluminum corrosion than some silver-coated fillers. Initially, this may seem contrary to the galvanic series chart, but there is a viable explanation.
While the galvanic series holds true for metals and metal alloys that contact each other, the behavior of core/shell particles follows a more complex galvanic coupling mechanism. In a paper presented at the 2004 IEEE/EMC Symposium,[1] it was demonstrated that core/shell particles take on the galvanic characteristics of the core material rather than the coating. Therefore, a nickel-coated graphite particle would behave more like graphite than nickel, and silver-coated aluminum would behave more like aluminum than silver.
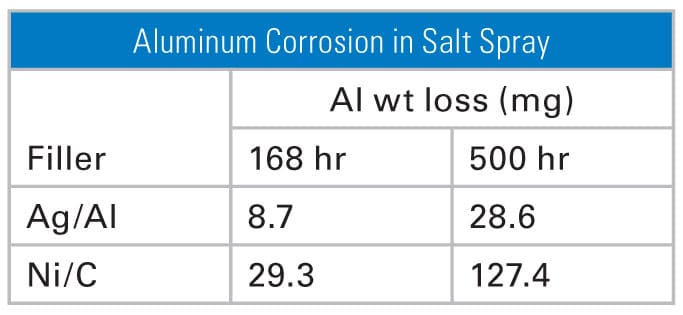
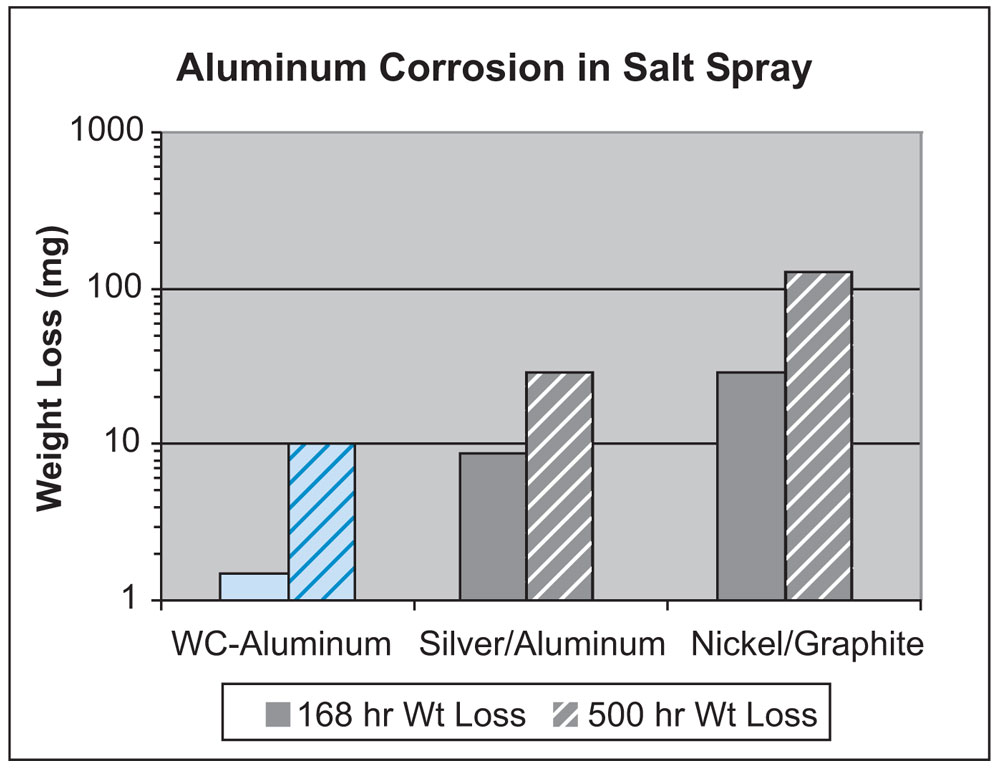
Salt spray corrosion tests made with fluorosilicone gaskets filled with silver-coated aluminum and nickel-coated graphite proved that the predicted behavior of the particles followed that of the core material. Aluminum corrosion, as measured by weight loss of an aluminum coupon in contact with the gaskets, was significantly higher for nickel/graphite than silver/ aluminum (Figure 2). This finding is explained by the fact that graphite is at the opposite end of the galvanic series from aluminum—thereby setting up a strong galvanic couple with an aluminum frame.
AN IMPROVED CONDUCTIVE FILLER
Based on earlier research, it would appear that the best conductive filler for a gasket that contacts aluminum would be aluminum. However, aluminum forms a natural oxide layer that is electrically resistive, which makes aluminum particles a poor choice for conductive fillers. Significantly, there is a technique for bridging the oxide layer of the aluminum particle to make the particles more conductive without adding a metal coating.[2]
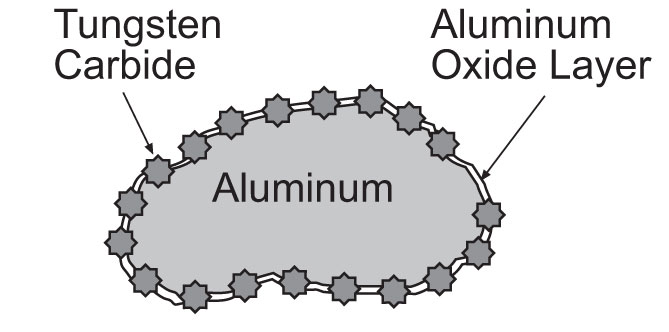
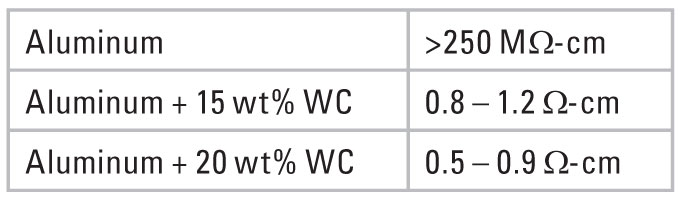
The unique particle has an aluminum core with a tungsten carbide coating. The tungsten carbide (WC) particles actually penetrate the aluminum oxide layer and act as multiple expressways for electron flow between aluminum particles (Figure 3). Tungsten carbide itself is not a metal, yet it has an electrical resistivity similar to stainless steel (70 μΩ-cm). Powder resistivity measurements show the effectiveness of this composite particle as compared to pure aluminum (Figure 4).
The best feature of tungsten carbide is that it has virtually no galvanic coupling with aluminum. It is a galvanically inert material. This allows the composite WC-Al particle to be extremely compatible with aluminum frames, as if aluminum were in contact with aluminum. Salt spray tests made with aluminum coupons in contact with fluorosilicone gaskets filled with WC-aluminum, silver/aluminum, and nickel/graphite confirm the superior compatibility of the WC-aluminum particle (Figure 5).
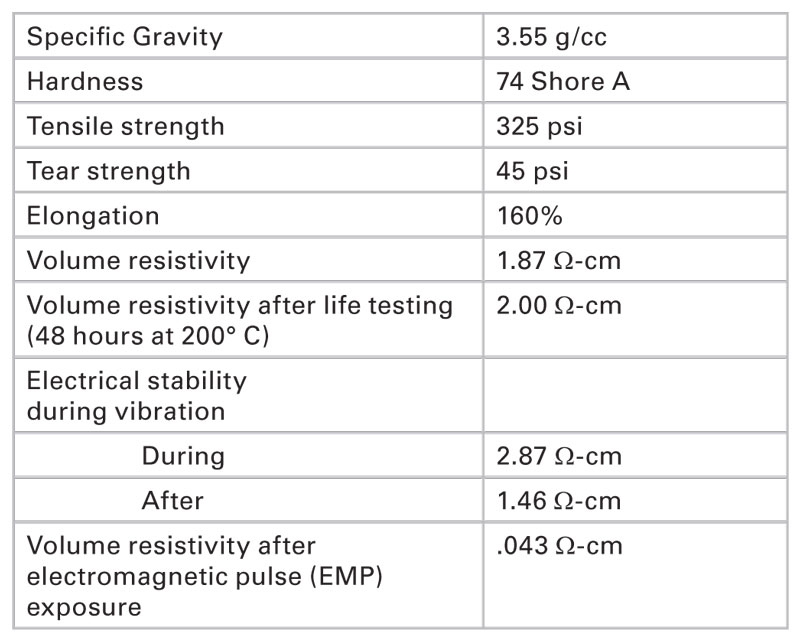
For a filler material to be of use in an EMI shielding gasket, properties other than aluminum compatibility are important. Gaskets filled with 70% by weight of a 45-μm diameter WC-aluminum filler were sent to an accredited testing laboratory for analysis. Test methods described in the standard MIL-DTL-83528C were used.
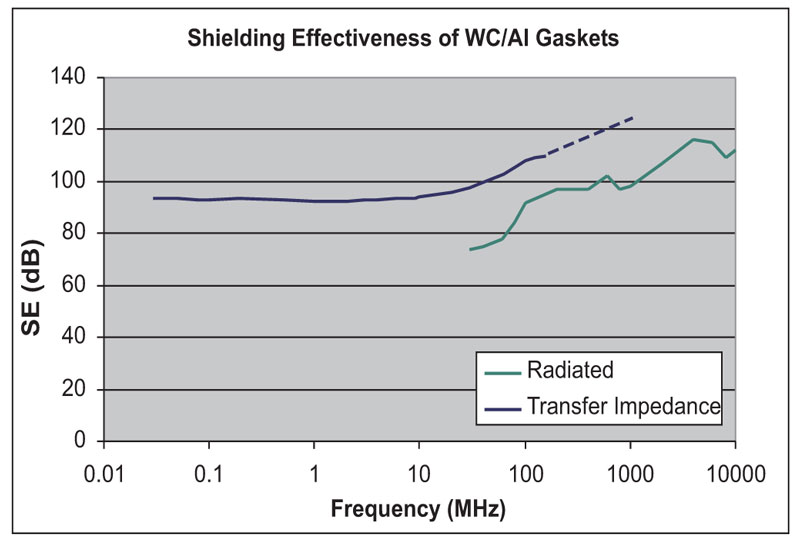
An interesting aspect of WC-aluminum filler is that it has excellent shielding properties even though it is not as conductive as silver-coated filler materials (Figures 6 and 7). Other materials, such as nickel/graphite, are also known to show this behavior.
Potential Uses of WC-Aluminum
Considering the combination of beneficial properties exhibited by WC-aluminum powder, creative product designers will no doubt find multiple uses for this unique material.
High corrosion environments
Marine environments are particularly severe high corrosion situations where almost any conductive metal in contact with aluminum will cause aluminum pitting. WC-aluminum would be ideal for these environments.
High sulfur environments
Silver is especially prone to reaction with environmental sulfur. The black silver sulfide that develops on the surface of a gasket filled with silver-coated filler will significantly increase its electrical resistivity. Situations in which exposure to fuel vapors is unavoidable would benefit from the non-silver filler, WC-aluminum.
Strong chemical exposure
Certain medical and military applications call for exposure to strong chemicals such as bleach for disinfection. Many metals are affected by this treatment. Tungsten carbide is a very chemically resistant material, and it enhances the chemical resistance of the composite WC-aluminum particle.
SUMMARY
Galvanic corrosion is a recurring problem with aluminum that product designers must face. The solution is to prevent the formation of a galvanic couple with aluminum in the first place. The conductive filler for EMI gaskets that has the least amount of galvanic corrosion on aluminum is a unique WC-aluminum composite particle.
REFERENCES
[1.] A.R. Pawlowych, “Galvanically compatible elastomeric gasketing material for EMI shielding applications,” 2004 IEEE/EMC Symposium.
[2.] R.J. Teichmann, “Galvanically compatible conductive filler,” US Patent 5,175,056.
