Testers seek to remedy the longstanding problem of “noisy’ responses from an EMG instrument.
Terence P. O’Laughlin and Michael J. Oliver, MAJR Products, Saegertown, Pennsylvania, USA
INTRODUCTION

This paper presents a shielding methodology and test in which an electromyography (EMG) instrument is shielded from spurious electromagnetic and radio frequency interference (EMI/RFI) emissions. These emissions are especially common in hospital settings such as operating rooms. Unfortunately, this phenomenon is also a frequent nuisance in clinics and medical office locations. Where EMI/RFI is present, it represents a very real hindrance to the accurate administration of both nerve conduction and EMG studies by any operator. Typical operators might include, but would not be limited to, electro-physiologists, physiatrists, neurologists, neurology technicians, neurosurgeons, and orthopedic spine specialists. EMI/RFI “noise” is an impediment to accurate testing because of the artifacts that interfere with the critical nerve responses within muscle tissue that is being evaluated for a variety of neurological conditions.


The authors’ attention was drawn to this problem in a decidedly non-clinical setting. Dr. Thomas O’Laughlin and his brother Terry O’Laughlin were discussing their occupations when Thomas realized that the shielding company where Terry is employed might be able to remedy the longstanding problem of “noisy” responses from an EMG instrument. Pursuing this thought, Dr. O’Laughlin and Terry set out to set up an electromagnetically clean environmental study that would address the issue of the artifacts that often interfere with medical professionals who are using and interpreting readings from an EMG machine in a EMI/RFI rife environment such as an operating room.
TEST LOCATION
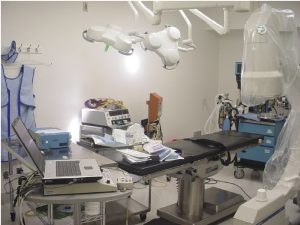
The electromagnetic study was performed at the California Industrial Rehabilitation Center in Fresno, California. Facility owner, Alvaro Valanzuela and his son Nico Valanzuela, were very helpful in facilitating the use of equipment and the outpatient surgical equipment operating room. Inside the surgical center, the electromagnetic study was set up and was performed in an operating room with typical instruments as seen in Figure 1.

The first stage of the test was to establish an electromagnetically quiet environment within the “noisy” operating room. To achieve this “electromagnetically clean” environment, the shielding products corporation coordinated the fabrication of a portable shielded conductive test enclosure (a Faraday cage) with dimensions of 10 ft. (W) x 8 ft. (L) x 8 ft. (H).
Shielded Enclosure

The shielded conductive tent enclosure is made of two silver conductive fabric layers. The tent is constructed using an external framework and is hung within the framework. (Figure 2 shows the left side and rear of the tent, and Figure 3 shows the front of the tent.) The conductive tent enclosure incorporated external power line filters that enable electromagnetically clean, 120-VAC power to be supplied within the tent enclosure. In addition, a separate and dedicated ground was utilized as shown in Figure 4.
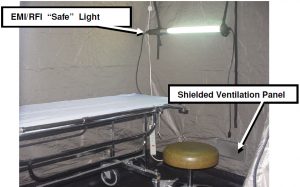
Inside of the tent, an electromagnetically shielded florescent light along with an operating table and chair was set up as shown in Figure 5. Ventilation was accomplished utilizing two shielded, tin-plated honeycomb panels (air vents). As shown in Figure 5, air was circulated inside of the tent using a fan that pushed air into the bottom air vent located on the right side of the tent. As depicted in Figure 2, air flowed out of the second air vent located on the top left side of the tent.
EMG MACHINE AND ELECTROMAGNETIC TESTING

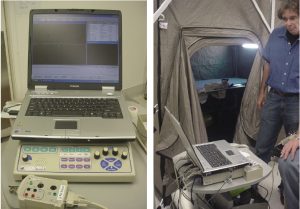
The instrument under test was an EMG machine. This instrument was calibrated. (See Figure 6.) An initial baseline and an actual reading outside the tent were needed. Taking on the role of patient, Terry O’Laughlin was tested to establish a initial reading obtained outside the shielded tent. See Figures 7 and 8.

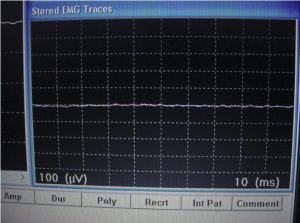
After establishing the baseline for EMG testing outside of the shielded tent, the EMG machine was moved into shielded tent, and the door was closed. All of the operating room equipment remained on, and the EMG parameters were set exactly as they had been during the initial test. Then, the EMG machine was recalibrated and was run through the same test as had been conducted outside the tent. Figure 9 shows the baseline response of the EMG machine inside the tent. As shown, this baseline response is very “clean.” Having been moved inside the tent, the EMG machine, which has been operating outside of the tent with 60-Hz filtering, could now produce a flat line response free of any artifacts produced by conducted or radiated electromagnetic interference.
After the calibration, Terry O’Laughlin was retested at the site of the same arm muscle using exactly the same leads. See Figures 10 and 11, and note the EMG machine reading obtained inside the tent. Clearly, the EMG machine results in Figure 11 are substantially easier to read than the previous test results shown in Figure 7.
CONCLUSIONS
The purpose of this study was to determine if the response of an EMG machine would improve in a shielded tent placed within an electromagnetically “noisy” environment. The outcome was clear. The success of this experiment was established by the “readability” of the trace on the EMG machine that had improved significantly with the elimination of electromagnetic noise (artifacts) that had been inter-modulated on the trace of the muscle response.
Particularly interesting were the response and subsequent comments by Dr. Thomas O’Laughlin. He as over 20 years’ experience with using an EMG device and performs testing an almost daily basis. He considered the response that had appeared on the screen when working outside the test normal and typical of the results he had obtained on a fairly regular basis in the surgical center.
Dr. O’Laughlin was astounded by the environment within the tent. First, Mike Oliver, an electrical engineer, and Terry O’Laughlin had Dr. O’Laughlin check his cell phone with the door to the tent open. He had four out of five “bars” showing cell phone connectivity strength. When the door to the tent was closed, he lost service completely, thus proving that the radiated frequency of 2.4 GHz needed to reach his phone had been completely and effectively blocked. Additionally, since the shielded tent is a passive device, it provides EMI/RFI attenuation over a very broad bandwidth that encompasses typical emissions from hospital equipment, commercial electronics, and broadcast stations.
As he began to calibrate the EMG machine inside the tent, Dr. O’Laughlin said, “There seems to be something wrong with the machine.” He re-started it three times until he realized that the flat line he was looking at was the true baseline as very, very little interference existed inside the tent. He told his brother Terry that the only other place he had ever seen that type of clean baseline was in a textbook—a remark capping off a very telling test on EMI/RFI and medical equipment.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr. Thomas O’Laughlin for his expertise and cooperation in conducting this experiment. They would also like to thank Alvaro Valanzuela and his son Nico Valanzuela for the use of their health care facility and equipment.
ABOUT THE AUTHORS
Terrence P. O’Laughlin is Vice President of Sales and Marketing at MAJR Products Corporation. He has a B.S. degree from Edinboro University of Pennsylvania. After long careers in education and sales, Terry joined MAJR and the EMC marketplace. Terry can be reached at terry@majr.com.
Michael J. Oliver has been an Electrical Engineer involved in Electromagnetics since 1990. Mike is currently Vice President of Electrical/EMC Engineering at MAJR Products Corporation in charge of customer EMC design and consulting and new product development, and is the ISO-9001:2000 management representative. His expertise is in EMI/RFI shielding technology with a background in electronics, military shelter electrical systems and high power antenna/radome design.
