Introduction
Essential Performance has been a mysterious term ever since its introduction in International Standard IEC 60601-1-2 in 2001. Essential Performance is not unique to IEC 60601-1-2, but originates in the general standard, IEC 60601-1, and is used throughout all the collateral and part 2 standards of the IEC 60601 family of standards for medical electric equipment, and medical electrical systems (medical devices).
Essential Performance
The Essential Performance of a medical device is determined by the following methods:
- The applicable IEC/ISO 60601-2-X standard for a device
- By the general safety standard 60601-1
- By the manufacturer as determined by risk analysis
Electromagnetic disturbances are only one phenomenon that could affect the device’s Essential Performance, or Basic Safety.
Immunity tests, such as Electrostatic Discharge (ESD) at 15 kV or 2kV power line surges, could affect the safety barrier dielectric characteristics, and must be considered as a safety risk.
The compliance criteria in IEC 60601-1-2 states that Essential Performance and Basic Safety must not be affected by electromagnetic disturbances. Basic Safety is defined in IEC 60601-1 and Part 2 standards.
One Part 2 standard identifies Basic Safety as unintentional or uncontrolled movement. It is important to include both Essential Performance and Basic Safety in the immunity compliance criteria.
Essential Performance could be thought of as safety related performance, the lack of which results in an unsafe medical device. More specifically, it is that performance of a device that will produce an unacceptable risk to the patient or operator if the absence of performance or degradation results in misdiagnosis or unacceptable harm.
IEC 60601-1, Edition 3.1 subclause 4.3, states the manufacture shall identify which function(s) of the medical device are essential for safety.
Note that Essential Performance is only related to safety of the medical device, and does not ensure that the device does what the manufacture specifies it will do.
The medical device can stop functioning so long as it does not create unacceptable risk to the patient or operator. Not every function of the medical device is essential for safety of the device.
Some examples of Essential Performance are listed below:
- The accuracy of a life-supporting function or correct administration of a drug, where incorrect administration would result in unacceptable risk to the patient;
- Correct operation of an alarm, where the failure to alarm would result in incorrect response by the medical personnel;
- Correct acoustic output power level of a diagnostic ultrasound imaging system, where excessive acoustic output power would result in unacceptable risk to the patient;
- Correct output of diagnostic information that is relied upon to determine treatment of the patient, where incorrect information would result in unacceptable risk to the patient;
- Correct or precise movement of a robotic catheterization machine by the remote controls, where unintended or uncontrolled motion of the catheter tip would result in unacceptable risk to the patient.
The risk management process is used exclusively in determining the Essential Performance of the medical device. The particular standards (IEC/ISO 60601-2-X) take precedence over the general safety standard (IEC 60601-1), and identify the Essential Performance for that specific medical device.
The following is an example of Part 2 Essential Performance requirements for Electrocardiographic Monitoring Equipment as specified in IEC 60601-2-27:
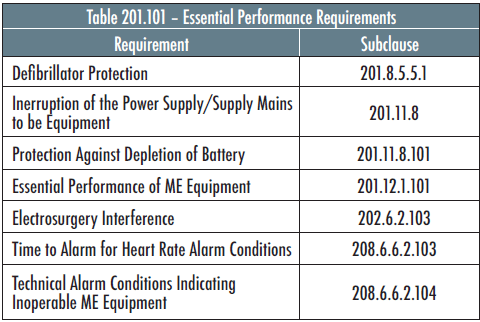
With the exception of defibrillator protection, the above Essential Performance parameters could be degraded by electromagnetic disturbances.
Probably the most interesting is 201.12.1.101, “Essential Performance of ME Equipment” which lists the following performance parameters as Essential Performance:
- Accuracy of signal reproduction
- Input dynamic range and differential offset voltage
- Input impedance
- Input Noise
- Multichannel crosstalk
- Gain control and stability
- Sweep speed
- Frequency and impulse response
- Gain Indicator
- Common mode rejection
- Baseline reset
- Pacemaker pulse display capability
- Rejection of pacemaker pulses
- Synchronizing pulse for cardioversion
- Heart rate range, accuracy, and QRS detection range
- Channel height and aspect ratio
- Tall T-wave rejection capability
The above list of Essential Performance parameters is daunting. Imagine how long it would take to test and verify all these parameters to meet the requirements of the standard during the 8 immunity tests required by IEC 60601-1-2.
A test plan (required by IEC 60601-1-2:2014) is vital to determine and justify which parameters of a device need monitoring during immunity testing.
Performance
What is performance of a medical device? Is it Essential Performance? The answer is simpler than the confusion of Essential Performance.
Device performance in the presence of electromagnetic disturbances is important because patients, operators and regulators have an expectation that the medical device will not only remain safe, but will work as the manufacturer advertised in the environment it will be used in.
For this article, we are only considering the electromagnetic environment. Performance of a medical device is determined by the manufacturer’s specifications and claims, and will may include the device’s Essential Performance as discussed below:
- The ability to print an ultrasound image remotely
- The ability of a scale to accurately measure patient weight
- The accuracy of X-ray tube voltage in X-ray equipment, e.g. the error is less than 5%
The device’s performance parameters must be monitored during immunity testing to ensure the effectiveness of the device per the manufactures specifications and claims.
Compliance criteria are the difference between Essential Performance and performance.
For Essential Performance, the device must remain safe as determined by the manufactures risk management process. For performance, the device must do what the manufacturer specifies and claims in the presence of electromagnetic disturbances.
Comparison of Essential Performance vs. Performance
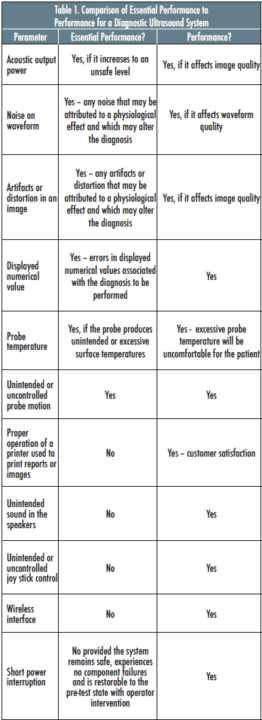
Table 1 provides a comparison of Essential Performance vs. the performance parameters for a Diagnostic Ultrasound System.
Summary
The concept of Essential Performance is often misunderstood. It is not the same as performance. Essential Performance is best understood as safety related performance.
A medical device’s Essential Performance, Basic Safety, and device performance, are all very important to customer satisfaction, regulators, device safety, and the manufacture’s quality system.
All should be monitored before, during, and after IEC 60601-1-2 immunity testing.
In addition, the requirements for the device’s instructions for use play a very important role in the device’s safe use and compliance with IEC 60601-1-2.